Kryospektroskopie
Der Schwerpunkt dieses Themas liegt in der Beobachtung einzelner, Fluoreszenz markierter Biomoleküle, die in einer sehr dünnen Schicht ihrer Lösung eingefroren sind. Durch diese Art der Immobilisierung ist es möglich, die Beobachtungszeit des Moleküls, von wenigen hundert Mikrosekunden in Lösungsexperimenten auf mehrere Sekunden zu erhöhen. Sie ist somit nur noch von der Überlebenszeit des Farbstoffmoleküls begrenzt, die jedoch, aufgrund reduzierter Photobleichprozesse, theoretisch länger ist als in Lösung.
Ziel beim Einfrierprozess ist die Erhaltung der "Raumtemperatur-Struktur" des Biomoleküls. Dies gelingt nur, wenn das Wasser beim Einfrieren vitrifiziert, d. h. in einen festen amorphen Zustand übergeht und keine Kristallstrukturen bildet. Entscheidend für die Vitrifizierung ist eine hohe Abkühlrate, ein geringes Probenvolumen und eine hohe Viskosität.
Für die Herstellung der dünnen Eisschicht wurde ein Kalt-Rotationsbeschichter entwickelt (siehe Abb. 1). In diesem wird ein sich drehendes Deckglas (ca. 9000 rpm) durch flüssigen Stickstoff gekühlt. Die Probe, Fluoreszenz markierte Biomoleküle in einer wässrigen Lösung, wird auf das Deckglas getropft und friert auf diesem fest. Anschließend wird das Deckglas in dem Dewar-Gefäß, in dem es gekühlt wurde, in einen Kryotopf, der auf einem Mikroskopstativ befestigt ist, transferiert und weiterhin mit flüssigem Stickstoff gekühlt (siehe Abb. 2).
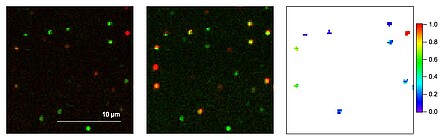
Mithilfe eines konfokalen Laser-Raster-Mikroskops werden die eingefrorenen Farbstoffe nun hinsichtlich ihrer Fluoreszenzeigenschaften, z. B. Intensität, Lebensdauer, Spektrum, Energietransfer, untersucht. Ist ein Biomolekül z. B. mit zwei Farbstoffen markiert, so kann die Effizienz des Förster-Energietransfers (FRET), die ein Maß für den Abstand zweier Farbstoffe darstellt, Aufschluss über die Konformation einzelner Moleküle geben (siehe Abb. 3).
Verena Hirschfeld

Gebäude 61
,
Raum 225
hirschfeld(at)physik.uni-luebeck.de
+49 451 3101 4207
Janosch Kappel

Gebäude 61
,
Raum 226
kappel(at)physik.uni-luebeck.de
+49 451 3101 4219